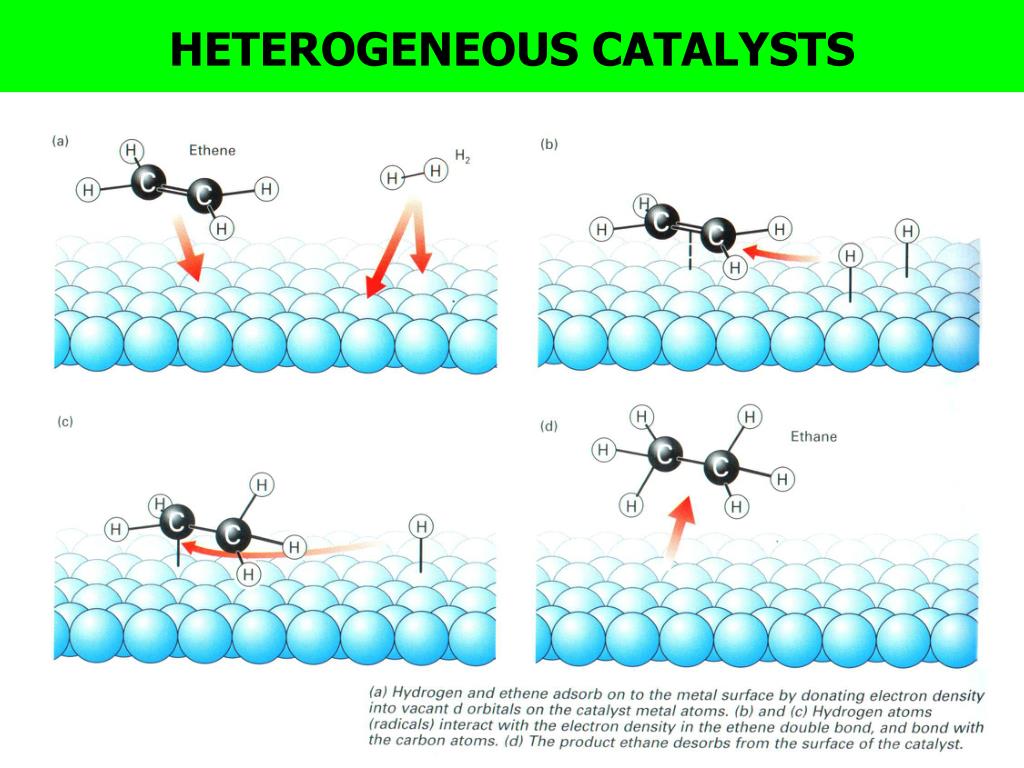
Heterogeneous Catalyst Kinetics . in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. aspects of kinetic analysis are reviewed taking into account different scopes of applied heterogeneous catalysis: At least one of the reactants interacts with the solid. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. The adsorption site is not always an active catalyst site, so. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were.
from www.slideserve.com
one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. At least one of the reactants interacts with the solid. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. The adsorption site is not always an active catalyst site, so. in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. aspects of kinetic analysis are reviewed taking into account different scopes of applied heterogeneous catalysis:
PPT Starter 1)Definition of catalysts 2) Difference between
Heterogeneous Catalyst Kinetics The adsorption site is not always an active catalyst site, so. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. aspects of kinetic analysis are reviewed taking into account different scopes of applied heterogeneous catalysis: In heterogeneous catalysis, the catalyst is in a different phase from the reactants. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. The adsorption site is not always an active catalyst site, so. At least one of the reactants interacts with the solid. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface.
From achs-prod.acs.org
Progress in Accurate Chemical Modeling, Simulations, and Heterogeneous Catalyst Kinetics in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. aspects of kinetic analysis are reviewed taking into account different scopes of applied heterogeneous catalysis: The adsorption site is not always an active catalyst site, so. in heterogeneous. Heterogeneous Catalyst Kinetics.
From www.slideserve.com
PPT Chemical Part 3 Reaction Mechanisms PowerPoint Heterogeneous Catalyst Kinetics In heterogeneous catalysis, the catalyst is in a different phase from the reactants. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. aspects of kinetic analysis are reviewed taking into account different scopes of applied heterogeneous catalysis: chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide. Heterogeneous Catalyst Kinetics.
From fr.slideserve.com
PPT 15 Chemical PowerPoint Presentation, free download ID Heterogeneous Catalyst Kinetics The adsorption site is not always an active catalyst site, so. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h. Heterogeneous Catalyst Kinetics.
From www.scribd.com
An InDepth Examination of Heterogeneous Reaction and the Role Heterogeneous Catalyst Kinetics one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. The adsorption site is not always an active catalyst site, so. in heterogeneous catalysis, reactants diffuse from the bulk. Heterogeneous Catalyst Kinetics.
From 2012books.lardbucket.org
Catalysis Heterogeneous Catalyst Kinetics chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. The adsorption site is not always an active catalyst site, so. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. in this chapter, two important kinetic equations, the arrhenius equation and the. Heterogeneous Catalyst Kinetics.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Heterogeneous Catalyst Kinetics in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic. Heterogeneous Catalyst Kinetics.
From www.mdpi.com
Catalysts Free FullText Heterogeneous Photocatalysis Recent Heterogeneous Catalyst Kinetics in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in this chapter, two important. Heterogeneous Catalyst Kinetics.
From exotukoyg.blob.core.windows.net
Catalyst Reaction Chemical Equation at Glenn Leopard blog Heterogeneous Catalyst Kinetics in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. The adsorption site is not always an active catalyst site, so. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. At. Heterogeneous Catalyst Kinetics.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID6391272 Heterogeneous Catalyst Kinetics one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. At least one of the reactants interacts with the solid. in this chapter, two important kinetic equations, the arrhenius equation and. Heterogeneous Catalyst Kinetics.
From www.cademix.org
Applications of Heterogeneous Catalysis in Industry Heterogeneous Catalyst Kinetics in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. At least one of the reactants interacts with the solid. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. The adsorption site is not always an active catalyst site, so. in the. Heterogeneous Catalyst Kinetics.
From www.researchgate.net
(PDF) Theory of the of Chemical Potentials in Heterogeneous Heterogeneous Catalyst Kinetics one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. At least one of the reactants interacts with the solid. in this chapter, two important kinetic equations, the arrhenius. Heterogeneous Catalyst Kinetics.
From www.slideserve.com
PPT Ch. 14Chemical PowerPoint Presentation, free download Heterogeneous Catalyst Kinetics In heterogeneous catalysis, the catalyst is in a different phase from the reactants. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. in the following sections, we focus on various factors controlling. Heterogeneous Catalyst Kinetics.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung Heterogeneous Catalyst Kinetics At least one of the reactants interacts with the solid. The adsorption site is not always an active catalyst site, so. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. in the following sections,. Heterogeneous Catalyst Kinetics.
From suncat.stanford.edu
Fundamental Concepts in Heterogeneous Catalysis Center for Interface Heterogeneous Catalyst Kinetics in this chapter, two important kinetic equations, the arrhenius equation and the reaction rate equation, were. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. in the following sections,. Heterogeneous Catalyst Kinetics.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Heterogeneous Catalyst Kinetics in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. aspects of kinetic analysis are. Heterogeneous Catalyst Kinetics.
From www.mdpi.com
Catalysts Free FullText Critical Review of LowTemperature CO Heterogeneous Catalyst Kinetics in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. in this chapter,. Heterogeneous Catalyst Kinetics.
From chemistry-europe.onlinelibrary.wiley.com
of Heterogeneous Single‐Site Catalysis Murzin 2023 Heterogeneous Catalyst Kinetics chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. in heterogeneous catalysis, reactants diffuse from the bulk fluid phase to adsorb to the catalyst surface. In heterogeneous catalysis, the catalyst is. Heterogeneous Catalyst Kinetics.
From www.youtube.com
OF BIMOLECULAR SURFACE HETEROGENEOUS CATALYSIS YouTube Heterogeneous Catalyst Kinetics one example of heterogeneous catalysis is hydrogenation of an unsaturated organic compound such as ethane (c 2 h 4) by. chemical kinetic modeling in heterogeneous catalysis is advancing in its ability to provide qualitatively or even quantitatively accurate. in the following sections, we focus on various factors controlling the intrinsic reaction kinetics of catalysts, and. in. Heterogeneous Catalyst Kinetics.